Abstract
Background Chronic graft-versus-host disease (cGVHD) is an important immunologic sequelae of allogeneic hematopoietic stem cell transplantation (HCT) that can lead to increased morbidity and mortality in long-term survivors. Red cell distribution width (RDW) is a measure of the variation of the mean corpuscular volume of red blood cells reflecting the degree of anisocytosis. Multiple epidemiological and longitudinal observational studies have shown that higher RDW values are associated with increased all-cause, cardiovascular and cancer-related mortality in different populations including patients as well as healthy participants but limited data exists in patients after HCT. Although RDW is affected to some degree by any type of anemia, the etiology of variation in RDW is considered to be multifactorial and overall incompletely understood. Whether RDW is affected by cGVHD activity or retains its prognostic significance for mortality is unknown.
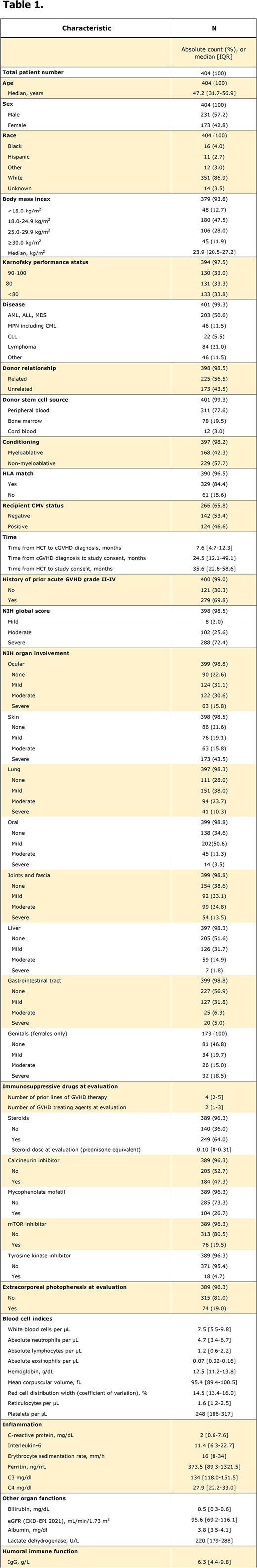
Patients and Methods The present study analyzed patients enrolled on the NIH cGVHD natural history study (NCT00092235) with existing measures of RDW (n=404). In addition to RDW, previously described laboratory markers of inflammation (Grkovic L. et al. Leukemia. 2012;26:633) significantly associated with cGVHD disease activity and other known prognostic factors associated with survival in cGVHD patients were considered.
Results The median age of the cohort was 47.2 years (IQR 32-57) and male patients were slightly overrepresented (n=231, 57%). Information about the NIH global score was available in 98.5% of patients who had predominantly severe (72.4%) and moderate (25.6%) cGVHD (Table 1). The median potential follow-up of the total cohort was 11.9 years (IQR 8.4-14.5) and the 5-year overall survival (OS) 77.9% (95% CI, 68.3-77.0%), but the median OS was not reached. A clear split was identified between the first quartile and the remaining overlapping quartiles of RDW distinguishing two groups with statistically different OS: <13.4% vs ≥13.4% (p=0.0024, adjusted for 3 implicit cut-points). Five-year OS was 84.1% (95% CI, 75.3%-89.9%) and 69.1% (95% CI, 63.6%-74.0%) for patients with RDW <13.4% and ≥13.4%, respectively. In univariate analyses, RDW ≥13.4% was statistically associated with Karnofsky performance status (KPS) <80 (39% vs 19%; p=0.0003), cGVHD global score severe (77.6% vs 62%; p=0.0046), increasing involvement of skin (p=0.0023) and mouth (p=0.0043), steroid (70% vs 46%; p<0.0001) and mTOR inhibitor treatment (24% vs 7%; p=0.0002), extracorporeal photopheresis (22% vs 10%; p=0.0077), increased inflammatory markers CRP, C3 and C4 (all p≤0.0002, respectively) and lower IgG (p=0.0031) but not age, sex, kidney function (eGFR CKD-EPI 2021) or time from HCT to study consent. The multiple logistic model established oral cGVHD (OR 1.77; 95% CI, 1.2- 2.60), mTOR inhibitors (OR 3.49; 95% CI, 1.5- 8.30), LDH (OR 1.01; 95% CI, 1.01-1.02), Hb (OR 0.68; 95% CI, 0.57-0.80) and ANC (OR 1.21; 95% CI, 1.07-1.36) as independent factors associated with dichotomized RDW groups. The Cox proportional hazards model confirmed the independent association of RDW ≥13.4% with worse OS (HR 1.61; 95% CI, 1.05-2.47) and identified albumin <3.45 mg/dL (HR 1.78; 95% CI, 1.41-2.24) and IgG <6.3 g/L (HR 1.67; 95% CI, 1.20-2.31) as independent factors in addition to KPS <80 (HR 1.51; 95% CI, 1.06-2.14), eGFR <45 mL/min/1.73m2 (HR 2.27; 95% CI, 1.41-3.66) and lung cGVHD NIH grade 3 (HR 2.92; 95% CI, 1.86-4.57). Moreover, RDW ≥13.4% was associated with decreased relapse-free survival (5-year; 72.8% vs 87.8%; p=0.0062 for comparison of the full curves) and increased non-relapse mortality (5-year; 20.8% vs 7.6%; p=0.0082 for comparison of the full curves) but not with cumulative incidence of relapse (5-year; 6.5% vs 4.6%; p=0.45).
Conclusion Patients with chronic GVHD and RDW ≥13.4% have a statistically worse overall survival due to higher risk of non-relapse mortality. Hypoalbuminemia and hypogammaglobulinemia — laboratory markers of increased inflammation and B-cell dysfunction — are additional independent poor prognostic factors identified by this study. It seems prudent that future therapeutic clinical trials should measure these laboratory markers to investigate whether they serve as surrogate biomarkers of chronic GVHD.
Disclosures
Schulz:Servier: Honoraria; Amgen: Consultancy, Honoraria. Pavletic:Center for Cancer Research at the National Cancer Institute: Other: Clinical Research Development Agreements with Celgene, Actelion, Eli Lilly, Pharmacyclics and Kadmon Corporation, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal